Equipment
Fluorescence correlation spectroscopy (FCS)
FCS measures diffusion, dynamics and spatial distribution of single fluorescent molecules in solution and in living cells.
Fluorescence correlation spectroscopy (FCS) measures diffusion, dynamics and spatial distribution of single fluorescent molecules in solution and in living cells. In order to observe the interaction between differently labeled molecules fluorescence cross-correlation spectroscopy (FCCS) is a very useful tool.
Principles FCS
Fluorescence correlation spectroscopy (FCS) monitors the relative fluorescence fluctuations in a small confocal volume element (light green in figure) which is typically less than 1 femtoliter. The fluorescence photons emitted from molecules in this volume element pass through a pinhole and are detected by a highly sensitive detector. The signal-to-noise ratio achieved by this method is very high, since signal interference from scattered laser light, background fluorescence and Raman emission can be largely eliminated. This allows measurements at the single molecule level. Diffusion of molecules, entering and leaving the volume element (see figure) is one of the most studied sources of fluctuation but also other processes like triplet state dynamics, isomerization, quenching or protonation of the chromophore have been studied. In the case of diffusion, the average time required for the passage of a single fluorescent molecule through the volume element is determined by its diffusion coefficient, which, in turn, is also related to the size of the molecule. Therefore FCS can be used to study the molecular interactions by observing a faster diffusing ligand and the slower diffusing complex.
Principles of Fluorescence Cross-Correlation Spectroscopy (FCCS)
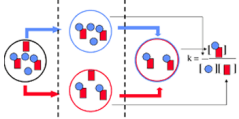
Fluorescence Cross-Correlation Spectroscopy (FCCS) differs from FCS that two detection channels are used, allowing to monitor two spectrally different fluorescent groups. In FCCS studies interacting molecules can be tagged by spectrally different fluorescent groups (f.e. blue and red dye). The molecular interaction can be studied by following the fluctuations in fluorescence intensity of both labeled molecules. In this case the discriminating factor for interaction is not the increase of molecular mass but the simultaneously occurring fluctuations in the fluorescence intensity. The spectrally different fluorescent dyes can be either excited with different lasers or with the same laser.The emission light is splitted into two different detectors by which the two fluorophores can be monitored simultaneously (figure). The cross correlation curve contains information about number and mobility of the interacting species. In contrast to FRET, it is not required that the two fluorophores are in very close proximity of each other.
Combining the high sensitivity and the possibility to monitor interactions makes FCCS suitable to study molecular interactions at physiologically relevant concentrations.
Equipment
For FCS, the Confocor 1 is available. It is based on an inverted Zeiss axio 100 microscope with a fixed beam. Three laser lines are available: 488, 514 and 543 nm.

The core of the FCCS-instrument, the ConfoCor 2 head (Zeiss), is integrated with the LSM510 (Zeiss) set-up, since it is coupled to the side port of the LSM microscope (manuals). Excitation light is provided by an argon ion laser(458, 488 and 514 nm) and two helium neon lasers (543 and 633 nm). A telescope system bundles the different laserlines into one beam. The intensity of each wavelength can be changed by using the Acousto Optic Tunable Filter (AOTF). The lasers are fiber-coupled into the ConfoCor2® head, which contains a set of appropriate excitation, dichroic and emission filters. Light is focused and collected by a water immersible objective lens (Zeiss C-Apochromat 40x, N.A 1.2) and detected by two fiber coupled avalanche photodiodes. The fluorescence intensity is autocorrelated by the AIM software (Zeiss-EMBL). The software also allows the storage of the complete photonstream in photon delay-mode. The data analysis is performed on a PC workstation with either the AIM software or with FCS Data Processor 1.2, an in-house developed software package which allows global fitting.
Publications
- Borst, J.W. & Visser, A.J.W.G. (2010). Fluorescence lifetime imaging microscopy in life sciences. Measurement Science and Technology, 21(10), 102002.
- Slootweg, E.J., Roosien, J., Spiridon, L.N., Petrescu, A.J., Tameling, W.I.L., Joosten, M.H.A.J., Pomp, H., Schaik, C.C. van, Dees, R.H.L., Borst, J.W., Smant, G., Schots, A., Bakker, J. & Goverse, A. (2010). Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. The Plant Cell, 22(12), 4195-4215.
- Zelazny, E., Miecielica, U., Borst, J.W., Hemminga, M.A. & Chaumont, F. (2009). An N-terminal diacidic motif is required for trafficking of the maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. The Plant Journal, 57(2), 346-355.
- Borst, J.W., Laptenok, S., Westphal, A.H., Kühnemuth, R., Hornen, H., Visser, N.V., Kalinin, S., Aker, J.C.M., Hoek, A. van, Seidel, C.A.M. & Visser, A.J.W.G. (2008). Structural Changes of Yellow Cameleon Domains Observed by Quantitative FRET Analysis and Polarized Fluorescence Correlation Spectroscopy. Biophysical Journal, 95, 5399-5411.
- Engel, R., Westphal, A.H., Huberts, D., Nabuurs, S.M., Lindhoud, S., Visser, A.J.W.G. & Mierlo, C.P.M. van (2008). Macromolecular crowding compacts unfolded apoflavodoxin and causes severe aggregation of the off-pathway intermediate during apoflavodoxin folding. Journal of Biological Chemistry, 283(41), 27383-27394.
- Hink, M.A., Shah, K., Russinova, E.T., Vries, S.C. de & Visser, A.J.W.G. (2008). Fluorescence fluctuation analysis of Arabidopsis thaliana somatic embryogenesis receptor-like kinase and brassinosteriod insensitive 1 receptor oligomerization. Biophysical Journal, 94, 1052-1062.
- Aker, J.C.M., Hesselink, R., Engel, R., Borst, J.W., Visser, A.J.W.G. & Vries, S.C. de (2007). In vivo hexamerisation and characterisation of the Arabidopsis thaliana AAA ATPase complex using FRET-FLIM and FCS. Plant Physiology, 145(2), 339-350.
- Zelazny, E., Borst, J.W., Muylaert, M., Batoko, H., Hemminga, M.A. & Chaumont, F. (2007). FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proceedings of the National Academy of Sciences of the United States of America, 104(30), 12359-12364.
- Aker, J.C.M., Borst, J.W., Karlova, R.B. & Vries, S.C. de (2006). The Arabidopsis thaliana AAA protein CDC48A interacts in vivo with the somatic embryogenesis receptor-like kinase 1 receptor at the plasma membrane. Journal of Structural Biology, 156(1), 62-71.
- Engel, R., Haastert, P.J.M. van & Visser, A.J.W.G. (2006). Spectral characterization of Dictyostelium autofluorescence. Microscopy Research and Technique, 69(3), 168-174.
- Nougalli Tonaco, I.A., Borst, J.W., Vries, S.C. de, Angenent, G.C. & Immink, R.G.H. (2006). In vivo imaging of MADS-box transcription factor interactions. Journal of Experimental Botany, 57(1), 33-42.
- Bosgraaf, L., Waijer, A., Engel, R., Visser, A.J.W.G., Wessels, D., Soll, D. & Haastert, P.J.M. van (2005). RasGEF-containing proteins GbpC and GbpD have different effects on cell polarity and chemotaxis in Dictyostelium. Journal of Cell Science, 118(9), 1899-1910.
- Bhat, R.A., Borst, J.W., Riehl, M. & Thompson, R.D. (2004). Interaction of maize Opaque-2 and the transcriptional co-activators GCN5 and ADA2, in the modulation of transcriptional activity. Plant Molecular Biology, 55(2), 239-252.
- Ruchira, A., Hink, M.A., Bosgraaf, L., Haastert, P.J.M. van & Visser, A.J.W.G. (2004). Pleckstrin homology domain diffusion in Dictyostelium cytoplasm studied using fluorescence correlation spectroscopy. Journal of Biological Chemistry, 279(11), 10013-10019.
- Russinova, E.T., Borst, J.W., Kwaaitaal, M.A.C.J., Yanhai Yin, Y., Caño-Delgrado, A., Chory, J. & Vries, S.C. de (2004). Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). The Plant Cell, 16(12), 3216-3229.
- Visser, A.J.W.G., Kunst, B.H., Keller, H.J.H.G. & Schots, A. (2004). Towards sorting of biolibraries using single-molecule fluorescence detection techniques. Current Pharmaceutical Biotechnology, 5, 173-179.
- Boteva, R., Koek, A., Visser, N.V., Visser, A.J.W.G., Krieger, E., Zlateva, T., Veenhuis, M. & Klei, I. van der (2003). Fluorescence analysis of the Hansenula polymorpha peroxisomal targeting signal-1 receptor, Pex5p. European Journal of Biochemistry, 270, 4332-4338.
- Hink, M.A., Borst, J.W. & Visser, A.J.W.G. (2003). Fluorescence correlation spectroscopy of GFP fusion proteins in living plant cells. Methods in Enzymology, 361, 93-112.