
Project
Putida for plastics
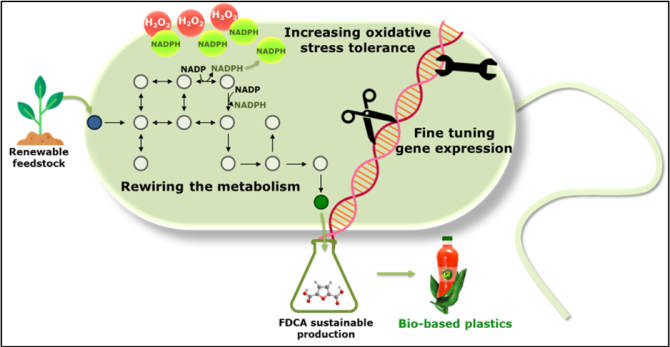
A major challenge of the 21st century is to meet rising global market demand for building blocks and polymers while ensuring environmental sustainability. White biotechnology has emerged as a promising green alternative, by relying on improved microorganisms for fuels and production of chemicals. Yet, substantial improvements are required to enable its economically feasible application to industrial processes. This project aims at tackling essential aspects of the metabolism and regulation of the microbe Pseudomonas putida to render it robust for industrial production of FDCA, a key precursor for the biodegradable polyethylene furanoate, which can become a major substitute of plastic.
Background
Despite human-accelerated climate change, the current global economy is still 80% dependent on fossil fuels, which are used for the supply of energy and production of building block chemicals and polymers. The urgent need to drastically move away from fossil fuels has boosted the regenerative circular economy. The Netherlands has committed to become 50 % circular by 2030. The use of microorganisms as cell factories to produce bio-based chemicals using renewable feedstocks holds the promise to strongly contribute to such a shift.
Pseudomonas putida is a soil microorganism with high potential for becoming an efficient cell factory:
- it has simple nutritional demands allowing fast growth in minimal medium,
- it shows astonishing capacity to host difficult redox reactions and to sustain environmental stresses and
- it is easily amenable to genetic manipulation.
Yet, the major challenge of using P. putida as cell factory for bio-industrial applications is to be cost-competitive with the petrochemical industry. Some of the limiting factors are the lack of robustness and full control over the regulatory network, as well as low productivities due to insufficient NADPH levels. How are we going to tackle these problems?
Project aims
- Fine- tune gene expression in P. putida by developing and deploying powerful genome editing tools
- Rewire its metabolism by simultaneously controlling multiple regulation levels
- Enhance its NADPH-dependent oxidative stress tolerance
Project description
There is a major challenge of using microorganisms as cell factories for bio-industrial applications, which is to be cost-competitive with the currently dominant petrochemical process. White biotechnology together with Synthetic Biology (SynBio) are slowly overcoming it by genetically improving microorganisms to significantly increase the yield and productivity of the target compound. The core of this field is to design cells, implementing robust, modular and standardized genetic pathways, which can be executed in a predictable fashion.
Among all microorganisms, Pseudomonas putida KT2440 has garnered special attention because it satisfies many of the needed requirements for becoming an alternative chassis to the laboratory workhorse Escherichia coli:
- simple nutritional demands allowing fast reproduction in minimal medium,
- easy genetic manipulation and
- an astonishing potential to host difficult redox reactions and to sustain environmental stresses.
Additionally, the diversity of substrate utilization and unique metabolic capabilities make P. putida an attractive candidate to overcome one of the main challenges found in industrial bioprocesses: inhibition of growth or productivity on particular feedstocks, high product concentrations or potential toxic intermediates. Different P. putida strains have been optimized to produce an impressive amount of valuable native and heterologous biotechnology products. However, to date, only 2-quinoxalinecarboxilic acid or 5-methylpirazine-2-carboxylic acid have been scaled up from the laboratory to the industry. So, why are not there more P. putida-based industrial processes?
For full industrial exploitation of its potential as a highly efficient cell factory for a wider range of biotechnological applications, the currently available tools and methods for precise and high throughput genome engineering are insufficient. Furthermore, robustness needs to be improved to create a controllable bacterial factory where interchangeable standardized genetic modules can be plugged-in and -out to deliver the predicted outcome while minimizing unwanted effects. Understanding and acquiring the full control over the regulatory network of cellular processes is the currently most important challenge to overcome, which would allow modelling and predicting the performance of the whole-cell chassis within its metabolic boundaries.
Main goals
In this project we aim at fine tuning gene expression in P. putida KT2440, rewiring its metabolism and increasing its reductive power to enhance FDCA productivity. To this end, we will:
- Create a library of synthetic modules (promoters, terminators and ribosome binding sites) to constitutively modulate gene expression: Tools for constitutive expression.
- Engineer riboswitches to fine-tune gene expression in response to external signals: Tools for inducible expression.
- Deploy CRISPR technology to simultaneously downregulate and upregulate native pathways.
- Increase the reductive power (NADPH) of P. putida KT2440 with the help of metabolic models to generate in silico predictions and the novel and powerful tools to experimentally validate them.
Publications
- M.Martin-Pascual, C. Batianis, L.Bruinsma, E. Asin-Garcia, L. Garcia-Morales, R.A.Weusthuis, R. van Kranenburg, V.A.M. Dos Santos (2021). A navigation guide of synthetic biology tools for Pseudomonas putida. Biotechnology Advances, 49, art. no. 107732. doi: 10.1016/j.biotechadv.2021.107732
- Corfee-Morlot, J., I. Westphal, M., & Spiegel, R. (2019). 4 Ways to Shift from Fossil Fuels to Clean Energy | World Resources Institute. Retrieved from https://www.wri.org/blog/2019/01/4-ways-shift-fossil-fuels-clean-energy
- Antink, R., Bakker, P., Coke-Hamilton, P., van Doorninck, M., Drinkwater, J., Dunlop, K., Eveillard, P., Haas, W., van Houten, F., Ishii, N., Katainen, J., Lopes Cardozo, M., Pantsar, M., Potočnik, J., Raworth, K., Saran, S., Steiner, A., Schmid, H., Sijbesma, F., Waughray, D., Webster, K., Wijkman, A., Watts, M. & Van Begin, G. (2019). Circularity Gap 2019. Retrieved from https://www.circularity-gap.world/
- European Commission. (2017). Circular Economy Research and Innovation - Connecting economic & environmental gains (pp. 10-11). Brussels: European Commission.
- Erickson, B., E. Nelson, J., & Winters, P. (2012). Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnology Journal, 7(2), 176-185. doi: 10.1002/biot.201100069
- Vickers, C., Blank, L., & Krömer, J. (2010). Grand Challenge Commentary: Chassis cells for industrial biochemical production. Nature Chemical Biology, 6(12), 875-877. doi: 10.1038/nchembio.484
- Roberts, M., Cranenburgh, R., Stevens, M., & Oyston, P. (2013). Synthetic biology: biology by design. Microbiology, 159(Pt_7), 1219-1220. doi: 10.1099/mic.0.069724-0
- Adams, B. (2016). The Next Generation of Synthetic Biology Chassis: Moving Synthetic Biology from the Laboratory to the Field. ACS Synthetic Biology, 5(12), 1328-1330. doi: 10.1021/acssynbio.6b00256
- Calero, P., & Nikel, P. (2018). Chasing bacterial chassis for metabolic engineering: a perspective review from classical to non-traditional microorganisms. Microbial Biotechnology. doi: 10.1111/1751-7915.13292