
Project
From feedstock to product and valorizable waste: Safe-by-Design in circular bioeconomy workflows
Industrial biotechnology is at the front line of a successful transition from a fossil-based economy towards a circular bio-based industry. However, a full circular bioeconomy workflow, from ‘waste’ being used as valuable feedstock for microorganisms and enzymes, to value-added and bulk products, to a valorizable excess, is still far from being fully established. Within this study we will examine one of the major bottlenecks to close the gaps of the sustainability transition: the incorporation of safety precautions throughout the entire biotechnological conversion process.
Background
For the sought circular economy, we require great efficiency of carbon usage and, in the long run, abstinence from virgin fossil feedstocks, along with integration of industrial end-of-life waste management. Industrial biotechnology uses microorganisms and enzymes to convert agricultural and other feedstock which are not suitable to serve as edible food into high value products with increasingly diverse applications and markets.
Synthetic biology is bound to be transformative for industrial biotechnology. The powerful methodologies for high-throughput genome engineering, model-driven design and circuit construction enable the development of tailored biocatalyst-based processes that would be otherwise impossible, unscalable or not economically feasible. Being based on engineering principles, synthetic biology also allows the design and incorporation of safety precautions into the entire biotechnological conversion process.
Non-conventional feedstocks arising from industrial and agricultural production and societal consumption may soon enable the manufacturing of multiple products from simple bulk chemicals to pharmaceuticals and materials through biotechnology. The development of this sector is proceeding rapidly and currently advances are made in replacing the use of refined sugars with cheaper complex feedstocks from agricultural side-streams to also allow the production of lower value (commodity) products.
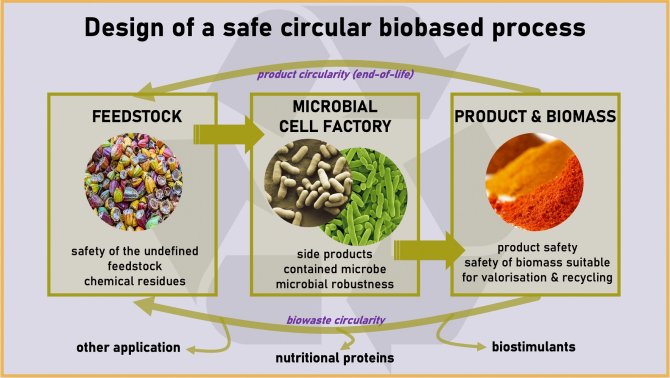
The quality of such less defined feedstocks poses challenges to the safety of the process. It has hardly been studied if and how fluctuations in less defined feedstocks and their contaminants affect the reproducibility of the microbial cell factory process. This is important to ensure safety of the product and preventing production of undesired toxic side products.
To close the cycle, the remaining biomass needs to be disposed or rather re-used in a safe way after the cultivation. With the anticipated strong growth of the sector, this poses an increasing (environmental and health) safety challenge. Projects on the deployment of microbial cell factories for aromatic products from waste streams have been and are ongoing at WUR, also in collaboration with industries, but the Safe-by-Design aspects of these processes and products have not yet been evaluated in an integrated manner.
Problem definition
A major question at stake in such research efforts is thus how to intrinsically embed safety and circularity aspects up-front and in every step of the process design. These include the safety evaluation of the feedstocks entering the production pipeline, the effect on quality and robustness of the pipeline itself and the safety of both products as well as wastes to allow reuse of left-over biomass after. Here, we aim to implement Safe-by-Design concepts in the industrially important Pseudomonas putida for production of aromatics and base chemicals from (waste) feedstocks.
Project description
As a show case, we will work on the microbial production, from agricultural wastes (lignin, glycerol) as feedstock, of aromatic compounds (curcumin, indole, cinnamic acid, adipic acid) as building blocks for a range of industrial applications. To this end, we will rely on the bacterium Pseudomonas putida as biocatalyst operating in bioreactor systems. We will pursue to valorise the waste streams from these bioreactor operations.
Furthermore, beyond the strictly technical aspects underlying this work, we will seek to embed the Safe-by-Design ethos along the development process. We will do so deploying reflexive models engaging the parties involved (researchers, connected companies, regulators, etc.; see report Schuurbiers, 2019).
Approach
- Inventarisation of critical steps, choices and risks that affect safety and circularity of production of selected food products and chemical building blocks through microbial conversion from waste feedstocks
- Reviewing the bacterial waste and safety aspects for reuse of the waste after bioreactor operation
- Experimental study on the fate of feedstock components and biocatalyst upon bioreactor operation
- Assessment of the different feedstocks on quality of the bioconversion process and potential uses of the off streams for further valorization
- Recommendations for embedding Safe-by-Design in circular bioeconomy workflows - feedstock to product and wastes
Publications
- Huijs, H., Asin-Garcia E., Roabey, Z, Martins dos Santos V. Towards an attitude for responsibility in safety education. Submitted
- Kallergi A, Asveld L. Perceptions of Safe-by-Design for biotechnology. Report to Ministry I&W, 2021
- Bouchaut, B. and Asveld L. Safe-by-Design: Stakeholders’ Perceptions and Expectations of How to Deal with Uncertain Risks of Emerging Biotechnologies in the Netherlands. Risk Analysis, Vol. 40, No. 8, 2020
- Kampers L., Asin Garcia-E. Martins dos Santos V. Navigating the Valley of Death: Perceptions of Industry and Academia on Production Platforms and Opportunities in Biotechnology. EFB Journal, Vol. 2, 2022
- Schuurbier D. Modellen voor een reflexieve component in technisch onderzoek gericht op Safe-by-Design. Report to Ministry I&W, 2019
- Schuurbier D. Over biotechnologie en veiligheid: Verslag van een serie interviews met onderzoekers binnen het NWO-TTW programma Biotechnologie en Veiligheid. Report to Ministry I&W, 2020